Blackford Analysis
LungPrint by VIDA provides quantitative support during COVID-19 Pandemic
With the world in the midst of an alarming pandemic, several Blackford Partners are coming forward with solutions to assist clinicians in their fight against COVID-19. One such partner is lung imaging analysis leader VIDA, who is offering their FDA -approved solution, LungPrint Discovery, to assist in the fight against the virus. Guidelines for the use of imaging with COVID-19 continue to evolve. If a chest CT has been obtained in a patient with suspected or confirmed COVID-19, LungPrint Discovery can provide quantitative imaging support and may help in the following ways.LungPrint Discovery in the context of COVID-19
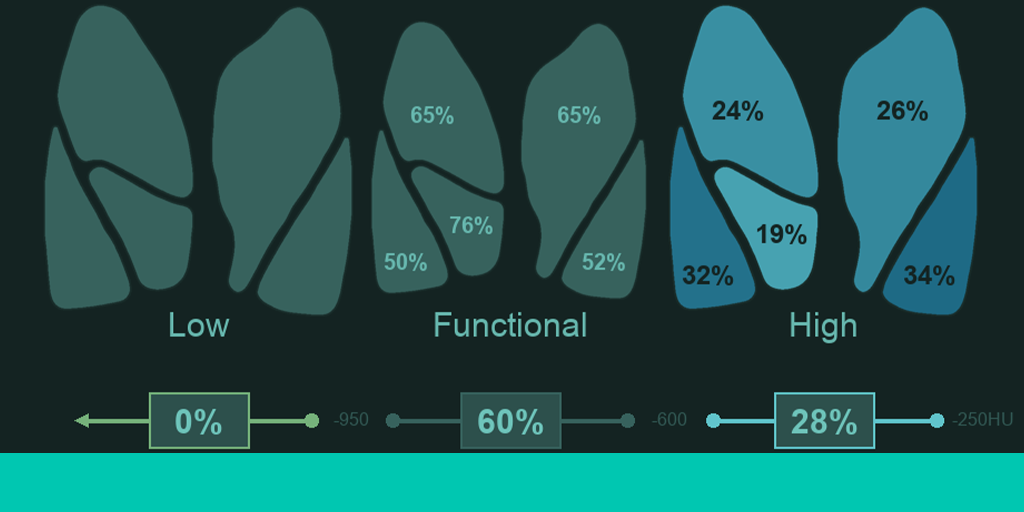
High Attenuation Area (HAA%) measured by LungPrint Discovery has been validated as a quantitative surrogate measure associated with interstitial lung abnormalities (ILAs) such as ground glass opacities, reticulations and fibrosis. Published studies on COVID-19 patients report imaging features consistent with ILAs, with typical findings including ground glass opacities with or without consolidation. Comorbid assessment of underlying conditions is indicative of increased vulnerability to disease progression with COVID-19. Quantitative support for assessment of underlying respiratory conditions may help providers better understand a patient’s risk profile for advanced disease and recovery. Quantitative longitudinal tracking of lung density and volume anatomically by lobe can be assessed on baseline and compared with follow up imaging. This comparison assists in more objective evaluation of disease progression and in monitoring patient response to treatment. How it works LungPrint Discovery includes an automatic and regional chest CT lung density report alongside a novel airway and lung visualization known as a topographic MPR. This combination provides a holistic view of the complexities of the lung for a rapid, global impression of lung health and disease. The holistic review is helpful to those who are both general and chest imaging specialists by increasing confidence and reducing fatigue during chest CT reads. LungPrint has been shown to reduce chest CT read times by 35%, providing workflow efficiency improvements and meaningful relief to radiology departments. Blackford’s view VIDA’s LungPrint Discovery can help clinicians evaluate features of COVID-19 and other underlying respiratory conditions affecting the patient's ability to breathe. This information can inform decision making on the care pathway for that patient. The LungPrint automation element is a key factor, especially in times of staff shortages when optimisation of resources is key. LungPrint is an established product; it’s a cleared medical device with a 510(K). Deploying LungPrint through the Blackford Platform minimizes IT investment for hospitals: deployment is through an existing, trusted relationship, without the need for a cloud connection. Using the Blackford Platform, eases LungPrint deployment, while minimizing legal review, business associate agreements, and training requirements. Get 4 months no charge use of VIDA LungPrint Discovery Enquire here to learn more about how to access VIDA LungPrint Discovery for 4 months at no charge: